Although water counts for 45-70% of an adult human’s body weight and is the most essential nutrient for survival, protein seems to be the critical macronutrient for body function. Our muscles, organs, blood cells, enzymes, hormones, and other bodily components are comprised of protein. Given this fact, it’s just as well that most people accept protein as a necessary part of the diet, unlike carbohydrates and fat which always seem to be at the center of some nutrition controversy. Whether you are a power lifter or a yogi, someone who follows a paleo diet or a vegan one, you likely recognize that protein is necessary to keep you healthy. So let’s talk a little bit about what it is, why your body needs it, and where to get it.
What is protein?
Proteins are large organic (i.e., carbon-containing) molecules that always contain nitrogen, the primary element that differentiates protein from carbohydrates and fat which are only made of various numbers of carbon, hydrogen and oxygen atoms. These nitrogen-containing molecules are sometimes called the “building blocks of life”, as they provide the structure for many parts of humans and other living organisms. From muscles to hormones to antibodies, our bodies are primarily made of and are kept functioning by protein molecules. However, the protein molecule is not the very basic structure. Amino acids are the molecules from which proteins are built.
Let’s explain proteins by beginning with the building blocks of the building blocks. Amino acids all have the same structure: an amino group, a carboxyl group, and an R group, where “R” indicates that this part of the molecule is different between amino acids.
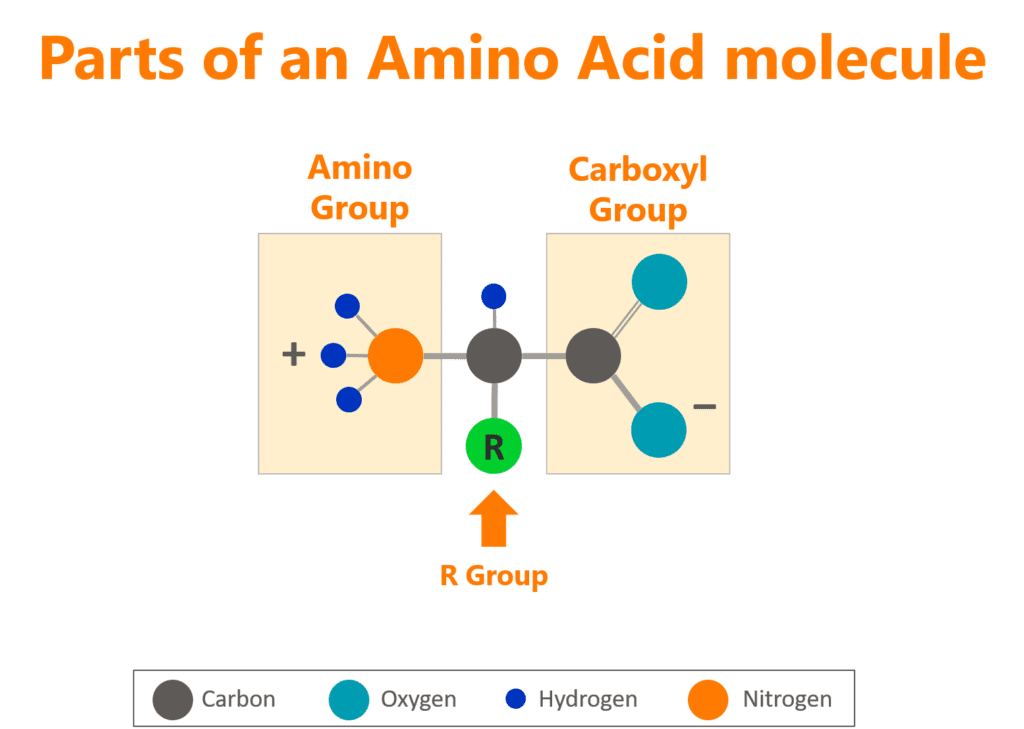
To create a protein, the amino group of one amino acid is bonded with the carboxyl group of another amino acid, in sequence, until all the necessary amino acids are bonded together in a long chain called a polypeptide chain.
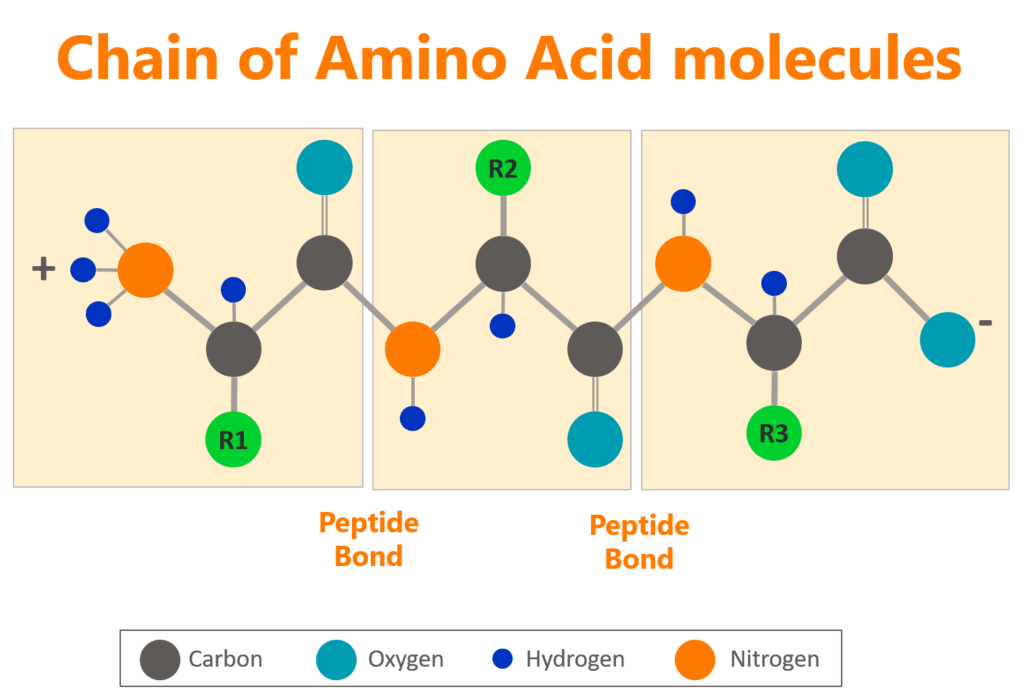
This polypeptide chain then folds itself into a unique 3-dimensional shape and bonds with other folded polypeptide chains to become a protein. Although we lump them all together in one group, each protein is unique—from which amino acids are chosen for the initial polypeptide chain all the way to the quaternary structure when two or more polypeptide chains bond together to create the final protein complex.
What happens to protein during digestion?
Upon ingestion, protein undergoes mechanical and enzymatic digestion. Chewing begins the process of protein digestion, but unlike carbohydrates, chemical digestion begins in the stomach when pepsin breaks the bonds within the protein complex and unfolds the polypeptide chain from its 3-dimensional structure. Upon leaving the stomach and entering the small intestine, the polypeptide chains (now part of chyme) encounter enzymes from the pancreas and small intestine that further break it apart.
Unlike carbohydrate bonds which are structurally unique, the bonds within protein are essentially the same since a nitrogen atom from one amino acid is connected to a carbon molecule in another amino acid. As such, the enzymes that break the peptide bonds tend to be specialized to specific locations in the long-chain of amino acids the protein is being broken or the type of amino acid needing to be separated. For example, carboxypeptidase breaks off the amino acid at the end of the polypeptide chain where the carboxyl group is facing “out” and aminopeptidase breaks off amino acids at the end where the nitrogen-containing amino group faces “out”. Chymotrypsin specifically works on bonds involving tyrosine, phenylalanine, and tryptophan, while elastase releases glycine, alanine and valine from the peptide chain.
Digestion is complete when the protein has been degraded to single or “free” amino acids, dipeptides (groups of two amino acid molecules), or tripeptides (chains of three amino acids). These protein fragments are absorbed and almost all are carried to the liver via the portal vein. The liver then has a few options of what to do:
- Break apart the dipeptides and tripeptides into individual amino acids
- Convert essential amino acids to non-essential amino acids (more on this later)
- Break down the amino acids to make glucose
- Use the amino acids to create proteins, including proteins needed by the liver or plasma proteins (like albumin) that are released into and have a job within the blood
- Release the amino acids into the blood stream where they can be taken up by various tissues throughout the body
The end of the digestive process yields a pool of amino acids that are available for the liver and other cells in the body to make bodily proteins.
What are essential vs. non-essential amino acids?
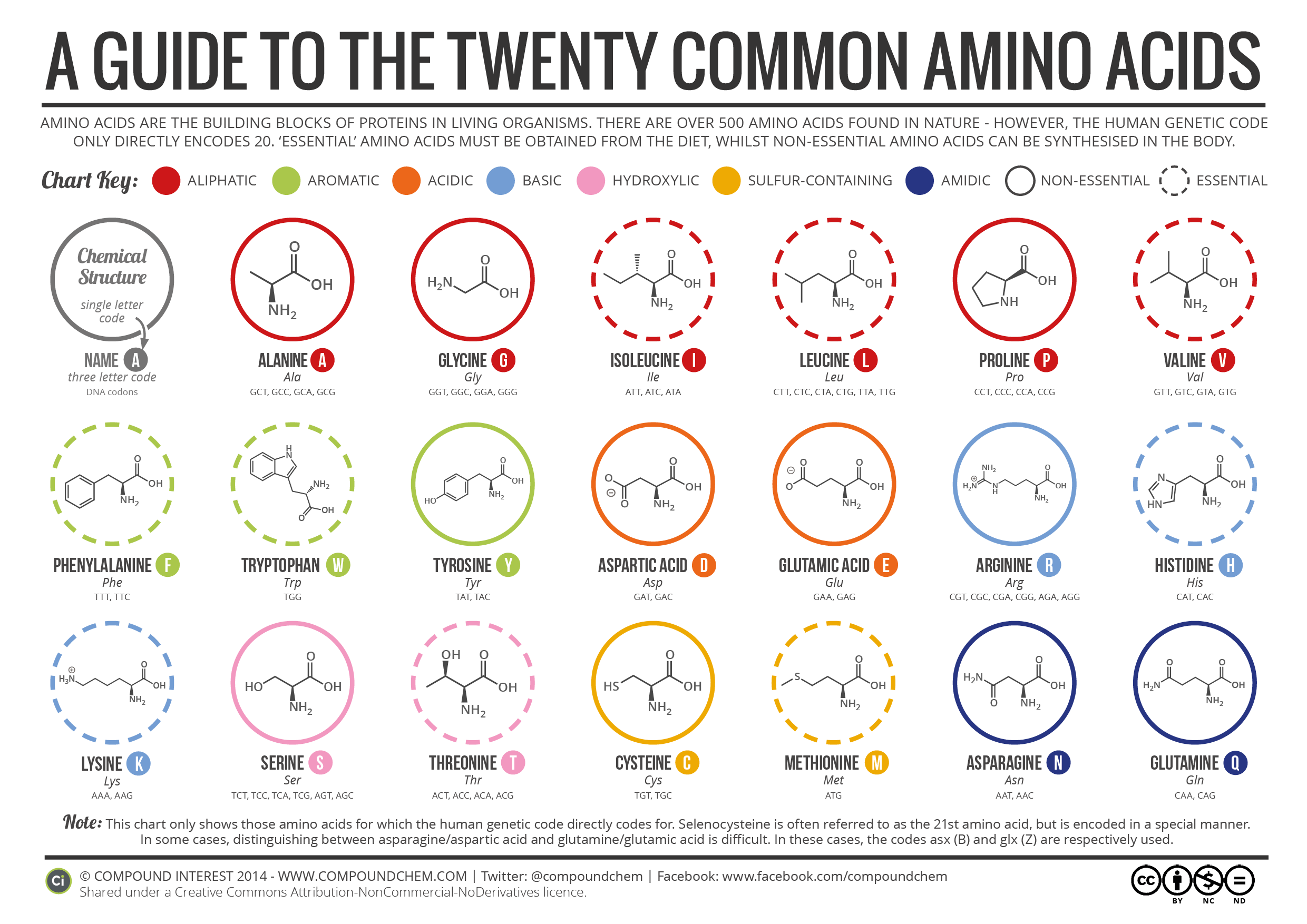
Although there are hundreds of amino acids in nature, there are only 20 that are required in the human body, meaning human DNA only includes these 20 in its instructions to make all of the proteins in our bodies. Of those 20, nine are essential amino acids, meaning that we must consume them in food as our bodies are incapable of creating them. An additional five are considered “conditionally essential”, meaning that the body can create them, but only if the organ that creates them is functioning properly. (Premature infants, people with liver disease, and those missing the necessary conversion enzymes need to ingest one or more of the five conditionally essential amino acids.) The remaining six amino acids can be consumed in food or can be created by the liver.
This beautiful graphic created by Compound Interest shows the 20 amino acids needed by humans. For each amino acid, you can see the carboxyl group represented by the lines with the “O” and the “OH” bonded together and the amino group represented by the line with the NH2. The R-group, as mentioned above, is the rest of the molecule, such as the pentagon at the end of the zigzag line in histidine or the zigzag line with an S in it making methionine.
How does protein in food become protein in the body?
Throughout the body, cells take in free amino acids or plasma proteins from the blood, add them to its internal amino acid pool, and use them to create new proteins needed by the specific cell. (Because I love that it has its own name, the process of recycling proteins within a cell is called ubiquitination. This process is responsible for repairing DNA, cleaning up dead cells, and mounting an immune response, among other things.)
That’s the simple answer. If you want the full biochemistry answer, read on…
As you remember from elementary school science, every cell in your body contains DNA. The DNA holds all of the information needed for your physical body to build and maintain itself. Essentially, our DNA is the library with all of the patterns needed to create every last part of us, which is mostly made of protein. Thus, when a cell needs to make a new protein, whether it’s a muscle cell because we’ve been lifting weights or an immune cell because we have a cold, the information about how to build that protein is first retrieved from the DNA.
Inside the cell’s nucleus where the DNA lives, RNA reads the pattern from the DNA and creates a copy within itself of the instructions. This newly informed RNA is now called messenger RNA (mRNA) and it carries the transcribed protein-making instructions out of the cell’s nucleus into the main part of a cell to hook up with a ribosome. The ribosome scrolls through the mRNA like an old 8-track ribbon and uses the instructions to tell transfer RNA (tRNA) which amino acid is needed next in the chain that is being created. tRNA grabs the necessary amino acid from the amino acid pool and delivers it to the mRNA/ribosome complex. While tRNA holds the amino acid in place, enzymes link the retrieved amino acid to the previously attached amino acid. When this is done, the tRNA releases to go retrieve another amino acid, if required.
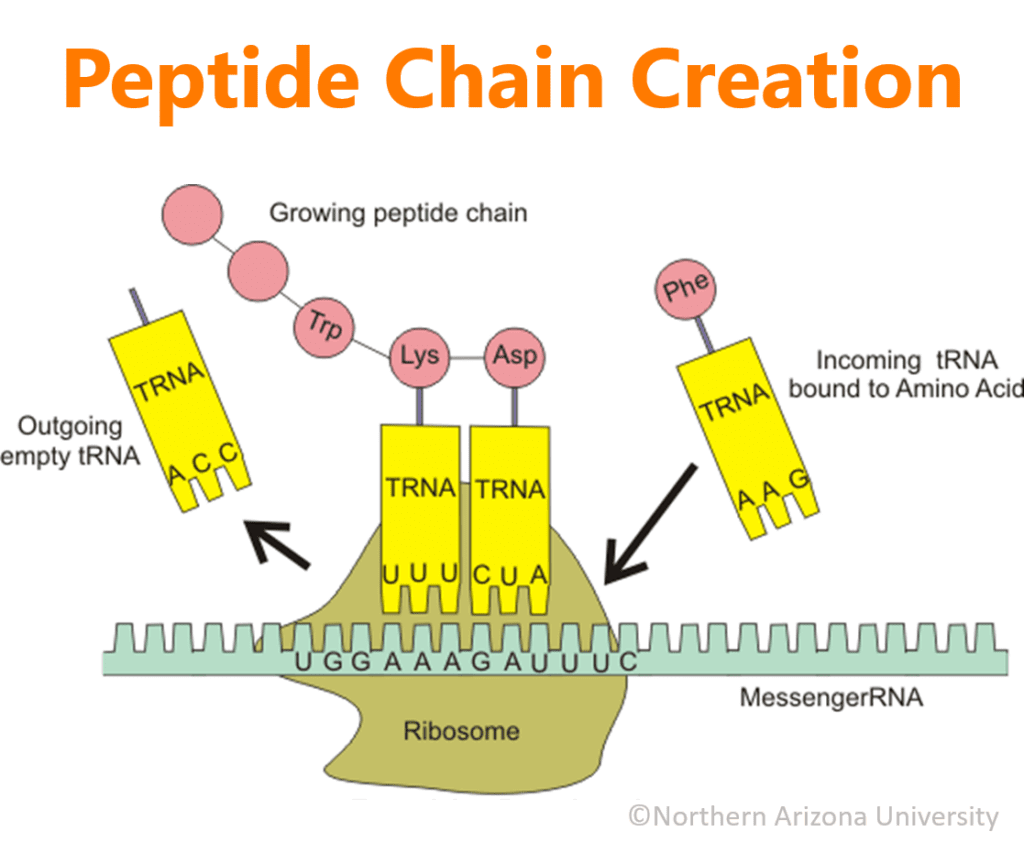
When the mRNA/ribosome complex reaches the end of the instructions, the polypeptide chain is released and then it usually travels to the Golgi apparatus (still within the cell) to be folded like origami or connected to other polypeptide chains until the desired final protein structure is achieved. When the production is complete, if the protein is needed outside of the cell in which it was produced, the Golgi organelle packages the protein into transport vesicles which will carry the protein outside of the cell, where it can then travel to its final destination.
Any amino acids that are not used in a relatively short period of time are returned to the liver for processing into something other than a protein as the body has no means outside of tissues, organs and muscles to store amino acids. This is another way that protein is unique from carbohydrates and fats, both of which can be stored in the body for future use.
What happens if we eat more protein than we need?
As mentioned above, the body does not store excess protein. If there is more protein in the diet than the body needs to create bodily proteins, the excess amino acids are transformed into something else.
The body can create at least some glucose from 18 of the 20 amino acids. Once the amino acids have been turned into glucose, that glucose can then be used for energy or stored for later use just like glucose from dietary carbohydrates. The other 2 amino acids can be turned into ketones and used for energy. If you are eating too much protein and have enough carbohydrate intake so that the body doesn’t need more glucose, the extra amino acids will be turned into fatty acids and stored as fat.
It is worth noting that whether the excess amino acids become glucose, ketones, or fatty acids, the nitrogen has to be stripped off of the amino acid as part of the transformation (remember: nitrogen is the main component of the protein molecule that differentiates protein from the other macronutrients). The removed nitrogen is processed to become urea or ammonia and is eliminated from the body, primarily through the kidneys as urine. If you’ve ever heard someone say that eating too much protein is hard on the kidneys, this is why… because the kidneys are responsible for eliminating the nitrogen byproducts from the body. However, only in those with pre-existing kidney disease is high protein intake potentially problematic.
When does the body break down its muscles?
The body is constantly breaking down old, worn-out or damaged proteins and creating (or synthesizing) new ones. For most children and adults, the amount of bodily protein that is degraded is essentially equal to that which is created when activity levels remain constant. For example, old red blood cells are degraded and new ones are formed; skin cells slough off as part of daily life and new ones are created; muscles are slightly damaged through daily activity and new muscle fibers are put in place.
Of course, there are exceptions to this and sometimes the body catabolizes (i.e., breaks down) more bodily protein than it makes. The following are a few of these situations:
- For older adults, their metabolic processes favor catabolism as a result of the aging process and they lose skeletal muscle more readily than their younger counterparts.
- During periods of starvation or prolonged periods of inadequate carbohydrate and fat ingestion, a person’s body will use some amino acids to create energy in the form of glucose or ketones.
- When the body is under metabolic stress, as might be experienced in serious conditions like sepsis (a life-threatening situation caused by an extreme infection) and severe injury or trauma (ex: severe burns), the body will break down less critical body proteins, such as skeletal muscle, to scavenge the amino acids.
In situations where your workouts are not as intense as they had been for a period of time, you will notice that your muscles get smaller. Your body is not eating up your muscles for energy, like it will glycogen or stored fat. Rather, the body breaks down the worn-out or damaged muscle fibers at the same rate it always has, but decreases the rate at which new muscle fibers are created by up to 50% because the muscle isn’t needed.
Generally speaking, so long as you are eating enough calories and/or have stored fat during hypocaloric (i.e., reduced calorie intake) time periods, the body will not use its own organs and muscles for energy.
What happens to undigested protein?
Just as our bodies are incapable of digesting all carbohydrates and fats, our bodies are not capable of digesting all proteins, with some people having more difficulty than others. For example, when babies are born, their digestive systems have not finished developing because they haven’t needed to digest anything while in utero. The inability to digest cow’s milk proteins is the most obvious manifestation, which can lead to constipation, diarrhea, or other gastrointestinal symptoms.
As we get older, our bodies usually develop enough enzymes and acids to fully digest whatever proteins we eat. But that isn’t always the case. People who have cystinuria are unable to absorb certain amino acids, while people with Crohn’s or celiac disease are unable to digest and absorb protein efficiently due to changes in the small intestine. As well, most people with a food sensitivity are intolerant of the protein within certain foods. For example, gluten is a protein found in wheat that humans cannot completely digest and causes various symptoms for some people, and casein is a protein in cow’s milk that takes a long time and multiple enzymes to digest, raising its potential for causing digestive discomfort.
Protein that isn’t digested into absorbable amino acids may cross into the blood stream via gaps in the small intestine wall (aka, Leaky Gut) or travels to the large intestine where it can be fermented by intestinal bacteria that are capable of consuming peptide fragments. Research has shown peptide-consuming bacteria are different species from those that consume digestion-resistant carbohydrates. Therefore, people who eat a higher protein diet will have a different gut microbiota composition than those who have a higher carbohydrate diet, and the excretions by those bacteria will also be different.
For some individuals, undigested protein has the potential to lead to a host of issues throughout the body from food allergies to insulin resistance to kidney disease. While all of the specifics are beyond the scope of this article, I strongly encourage anyone who experiences adverse effects from eating protein and/or believes they are protein intolerant to contact a registered dietitian or other healthcare practitioner to help them navigate their own physiology and find a personalized way of eating that fully nourishes their body.
Which foods contain protein?
Protein is readily available in our food supply from both animal and plant-based foods. However, not all protein foods contain all of the amino acids that the body needs to make muscles and other tissues. Animal sources of protein, such as beef, poultry, fish, eggs and some dairy products, contain all of the essential amino acids and, therefore, are considered “complete” proteins. Any food which does not contain all of the essential amino acids is considered an “incomplete” protein.
This is by no means to say that you must be a meat eater to get all of the amino acids that you need! Almost all plant foods contain at least some protein, with fruits containing the smallest amount. Of course, the protein in plant foods is not as easily accessible as the protein in animal products because it is wrapped in carbohydrate, which gives plants their structure. Even so, eating a variety of whole plant foods allows the vegetarian or vegan to easily ingest all the required amino acids and ensure their availability in the amino acid pool. For vegetarians, eggs and dairy are great sources of all the essential amino acids. For vegans, quinoa, buckwheat, hempseed, chia seeds, soybeans and Ezekiel bread contain all of the essential amino acids.
Although your body needs all of the essential amino acids in order to properly build all the proteins in your body, remember that you don’t have to get all of them in each bite… you just have to get enough each day to keep your amino acid pool populated with the ones needed to maintain your body.
Conclusion
In summary, protein is an indispensable nutrient that plays a fundamental role in almost every physiological process in the body. From building muscle and supporting immune function to producing enzymes and hormones, protein is essential to our survival and well-being. Understanding the journey of this macronutrient from our diet to its final roles in the body can help us appreciate the importance of consuming a balanced diet rich in high-quality protein sources. Whether you’re aiming for muscle growth, weight management, or overall health, ensuring adequate protein intake is key to achieving your goals.
Notes:
- Some people are incapable of using certain amino acids after they have been absorbed. People with metabolic disorders such as phenylketonuria (PKU) and tyrosinemia must follow specialized patterns of eating in order to manage these conditions.
- How much protein an individual needs should be determined based on their age, activity level and health conditions. It’s a fairly big topic and will be covered in another post!
Image by RitaE on Pixabay.
Sources:
— Baker TE, Zopey M. The role of antioxidants in preventing cardiovascular disease. In: Textbook of Cardiovascular Medicine. 3rd ed. Springer; 2022. https://www.ncbi.nlm.nih.gov/books/NBK562306/. Accessed August 13, 2024.
— Bertolotti M, Prandi B, Casini V, et al. Nutrition in diabetic retinopathy: A review. Nutr Diabetes. 2021;11(1):34. doi: 10.1016/j.nutd.2021.100083.
— Byerley L. Protein digestion, absorption, and metabolism. LibreTexts website. https://med.libretexts.org/Courses/American_Public_University/APUS%3A_An_Introduction_to_Nutrition_(Byerley)/APUS%3A_An_Introduction_to_Nutrition_1st_Edition/05%3A_Proteins/5.04%3A_Protein_Digestion_Absorption_and_Metabolism. Updated August 8, 2023. Accessed July 14, 2024.
— Compound Interest. A Guide to the 20 Common Amino Acids. Accessed on March 10, 2015. http://www.compoundchem.com/wp-content/uploads/2014/09/20-Common-Amino-Acids-v3.png
— English N. 12 Complete Proteins Vegetarians Need to Know About. Accessed on March 10, 2015. http://greatist.com/health/complete-vegetarian-proteins
— Gropper SS, Smith JL. Advanced Nutrition and Human Metabolism. 6th ed. Belmont, CA: Wadsworth Cengage Learning; 2013.
—LibreTexts. Protein digestion, absorption, and metabolism. LibreTexts website. https://med.libretexts.org/Courses/American_Public_University/APUS%3A_An_Introduction_to_Nutrition_(Byerley)/APUS%3A_An_Introduction_to_Nutrition_1st_Edition/05%3A_Proteins/5.04%3A_Protein_Digestion_Absorption_and_Metabolism. Updated November 6, 2022. Accessed July 14, 2024.
— Mahan LK, Escott-Stump S, Raymond JL. Krause’s Food and the Nutrition Care Process. 13th ed. St. Louis, MO: Elsevier Saunders; 2012.
— Morrison JL, O’Donnell C, Shennan AH. The role of maternal diet in the development of fetal programming. J Physiol. 2015;593(22):4811-4823. doi: 10.1113/JP270083.
— National Center for Biotechnology Information. Epigenetics: The role of genetics in health and disease. In: Molecular Biology of the Cell. 6th ed. Garland Science; 2014. https://www.ncbi.nlm.nih.gov/books/NBK556052/. Accessed July 16, 2024.
— National Library of Medicine. How genes work: making a protein. MedlinePlus Genetics website. https://medlineplus.gov/genetics/understanding/howgeneswork/makingprotein/. Updated May 30, 2023. Accessed July 14, 2024.
— Protein synthesis. Northern Arizona University website. https://jan.ucc.nau.edu/lrm22/lessons/protein_synthesis/protein_synthesis.htm. Updated August 10, 2023. Accessed August 13, 2024.